SPPS 208: Study Design & Biostatistics II (Mark Bounthavong & Kathryn Hollenbach (WI21)/ Zaid Yousif (WI22/WI23/WI24), UCSD)
Schedule: Winter 2021 / Winter 2022 / Winter 2023 / Winter 2024
Description: This is the second part of a two-part course series on Study Design and Biostatistics. Students will build on their study design and biostatistics foundation from the first quarter to complete a research proposal and learn how to respond to a reviewer and editorial critiques of their proposal. Students will learn more complicated biostatistical methods used to answer their associated scientific questions as well as to write a detailed analysis plan of their research proposal. Additionally, students will, ultimately, become proficient in writing research abstracts and critiquing the scientific literature.
Students will continue using STATA statistical software (College Station, TX) to manipulate data sets, analyze pharmaceutical data, and understand the application and interpretation of statistical analyses. Students will become proficient in conducting multivariate analyses, synthesizing output and presenting data results as well as understanding the limitations of respective data sets and analyses.
Course Objectives
Critically assess the biomedical literature for study design, confounding, and missing data.
Estimate sample size and power for a study
Compare and contrast types I and II errors
Know the different methods for handling missing data or patients lost to follow-up
Understand the differences between a confounder and interaction
Identify and handle confounding through study design and statistical analyses
Write a robust Methods section to answer a testable research question for a research proposal
Write a research proposal for a clinical study related to medication(s) or pharmacy practice, including study elements discussed in class.
Write a robust analysis plan to answer a testable hypothesis for a biomedical study
Critically assess medical, pharmacy, and healthcare literature to make clinical or practice decisions across clinical practice settings.
Be able to identify and perform the appropriate statistical test(s) for specific types of data.
Understand the different multivariate biostatistical tests and their application to pharmaceutical data.
Be able to draw appropriate conclusions based on analysis results.
Be proficient in the use of STATA statistical software for data analysis.
Be proficient in understanding data output.
Understand the use of and limitation(s) of individual data analyses.
Gain proficiency in critically evaluating scientific research.
Synthesize criticisms and reviews to improve project quality.
SPPS 206: Study Design & Biostatistics I (Mark Bounthavong & Kathryn Hollenbach (FA20)/zaid yousif (FA21/FA22/FA23), UCSD)
Schedule: Fall 2020 / Fall 2021 / Fall 2022 / Fall 2023
Description: This is the first of a two-part course series on Study Design and Biostatistics. This course series will introduce students to common study designs used in clinical sciences and the biostatistical methods used to answer their associated scientific questions. Throughout the series, students will be provided examples from the literature and develop an understanding of what type of statistical tests are appropriate to specific study designs and scenarios.
This course will focus on examining and designing different types of studies that influence medication use and decision-making in pharmacy practice settings. Types of study designs range from randomized controlled clinical trials (RCTs) to observational and evaluation studies. Not all possible study designs will be covered in this course series; however, highlights of common designs relevant to translational research in pharmacy practice will be reviewed in class.
Additionally, this course will explore biostatistics concepts, statistical tests, and appropriate application of statistical methods relevant to pharmacy and medicine. Students will learn to use STATA statistical software (College Station, TX). STATA16 will be used to manipulate data sets, analyze pharmaceutical data, and understand the application and interpretation of statistical analyses. Students will become proficient in conducting analyses, synthesizing output and presenting data results as well as understanding the limitations of respective data sets and analyses.
Course Objectives:
List study design elements (design, sample, data, dependent and independent variables, time period, analytic methods, etc.) that provide evidence for therapeutic decision-making
Compare and contrast major types of study designs used in clinical health sciences
Understand ethical issues associated with conducting clinical studies by completing the CITI human subjects human training
Critically assess medical, pharmacy, and healthcare literature to make clinical or practice decisions across clinical practice settings
Write a research proposal for a clinical study related to medication(s) or pharmacy practice, including study elements discussed in class
Understand the different univariate biostatistical tests and their application to pharmaceutical data
Be able to identify and perform the appropriate statistical test(s) for specific types of data
Be able to draw appropriate conclusions based on analysis results
Be proficient in the use of STATA statistical software for data analysis
Become proficient in understanding data output
Understand the use of and limitation(s) of individual data analyses
Gain proficiency in critically evaluating scientific research
SPPS 209: Applied Pharmacoeconomics (Mark Bounthavong (SP20, SP21, SP22, SP23), UCSD)
Schedule: Spring 2020 / Spring 2021 / Spring 2022 / Spring 2023
Description: Applied Pharmacoeconomics is an introduction to pharmacoeconomic principles used to manage drug therapy for patient populations and to produce quality clinical, economic and humanistic outcomes in a cost-effective manner. Course emphasizes application of basic management and decision analysis functions to evaluate the need for and document the effect of pharmaceutical care interventions in a variety of healthcare practice settings.
Course Objectives:
Identify whether a strategy is cost-effectiveness using the cost-effectiveness plane
Determine which strategy is cost-effectiveness using the principles of extended dominance and the cost-effectiveness frontier
Identify and explain the different types of pharmacoeconomic analyses (e.g., cost-effectiveness, cost-utility, cost-benefits, cost-minimization analyses)
List the different types of perspectives used in pharmacoeconomic analyses
Determine the most appropriate pharmacoeconomic analysis to use for a specific scientific question or setting
Identify and explain the different types of costs (e.g., direct, indirect, and intangible)
Incorporate costs and benefits into a pharmacoeconomic analysis
Use external data to inform a decision analysis model (data synthesis)
List the advantages and disadvantages of using the quality adjusted life years (QALYs) in pharmacoeconomics
Distinguish between a deterministic and stochastic model
Identify when to use a decision tree versus a Markov model
Calculate the incremental cost-effectiveness ratio using costs and benefits
Conduct a cost analysis by constructing a decision model (decision tree, Markov model) using data from the literature and public sources (e.g., American Medical Association, Veterans Affairs)
Critique a pharmacoeconomic analysis or study
HSMGMT 523: Informatics in Healthcare Management (Ian Randall, University of Washington)
Schedule: Fall 2018 / Winter 2019 / Fall 2019
Description: Medical informatics concerns the representation, organization, and manipulation of biomedical information and knowledge. Exposes students to a high-level understanding of informatics and its health care applications. Discussion of successes and failures in implementing information technology focuses on gaining leadership and management knowledge that embraces informatics.
Summary: This lecture focused on using Tableau to generate dashboards using the classic AdventureWorks database. I lectured on clinical business intelligence and the elements of relational database management and data visualizations. There were approximately 40 students from the Masters of Healthcare Administration program.
PDF version of my slides are available here.
Optional readings are available here.
Tableau tutorial and exercise are available here.
Link to video tutorial is available here.
PHARM 592 Pharmacy Practice IV: Design and Analysis of Medical Studies (Emily Beth Devine, University of Washington)
Schedule: Fall 2018
Description: Introduces the basic biostatistical concepts used in the medical literature, and the various study designs. Develops students' skills in critically evaluating the medical literature, with the goal of applying these skills to clinical practice.
Summary: I lectured on the chi square distribution and test. I also had the students follow a few examples and demonstrated how a simulated chi squared distribution using a d20 dice could be biased. There were approximately 100 second-year pharmacy students in the class.
Exercises (Excel and R files) are located here.
I wrote a blog about using the chi square test on a d20 dice.
R code for exercises can be found on my GitHub site.
PHARM 542 - Managed care pharmacy: principles and practice (Pete Fullerton and David Veenstra, University of Washington)
Schedule: Fall 2018 / Fall 2019
Description: Surveys the activities, tactics, and strategies used by managed care to deliver pharmacy services to their members. Includes: formulary development, clinical improvement programs, quality improvement measures, regulatory activities, contracting with pharmaceutical manufacturers, network management, financial issues, sales and marketing, and provider relations.
Summary: I lectured on the VHA’s formulary process and system to PharmD students. I also provided an exercise to identify whether adalimumab was on the VHA’s formulary and its costs compared to certolizumab, a comparator. Class size was approximately 15 pharmacy students.
PDF version of my slides are available here.
Optional readings are available here.
UCONJ 599 - Selected Readings in Interdisciplinary Clinical Research (Patrick o'keefe and Stacey Long-Genovese, university of Washington)
Schedule: Summer 2018
Description: Analysis and synthesis of selected readings and works in progress related to multidisciplinary and interdisciplinary clinical research. Class focused on training scientists in the methods and philosophy of conducting clinical translational research. The Institute of Translational Health Sciences is dedicated to speeding science to the clinic for the benefit of patients and communities throughout Washington, Wyoming, Alaska, Montana, and Idaho.
Summary: I gave a lecture at the Institute of Translational Health Sciences to the TL1 trainees on the principles of Comparative Effectiveness Research (CER). This includes a backing on CER, outcomes research, and study designs. Class size was approximately 20 students (medical, pharmacy, engineering, and public health).
PDF version of my slides are available here.
Optional readings are available here.
PHRM 8400 - Health-Systems Practitioner Track: Advanced elective (James D. Scott, Western University of Health Sciences)
Schedule: Winter 2018 / Winter 2019 / Winter 2020
Description: The Health-Systems (clinical/research) Practitioner Track is intended for student pharmacists interested in furthering their clinical training in settings such as hospital, ambulatory care, home infusion, skilled nursing facilities and other non-community practice sites. This includes a dedicated research project and poster presentation of their work, which may include medication use evaluations, staff education guidelines, cost-effectiveness analyses, implementation of pre-printed order sets, etc. Student pharmacists should expect to be on campus for orientation, the Board Prep/PIC Week and AE Poster Day.
Summary: I provided a review of clinical study designs and statistical support for the fourth-year PharmD students. Lectures include an overview of study designs and a review of basic statistical inference. Topics include a comparison of different study designs (cross-sectional, cohort, and randomized controlled designs), internal and external validity, statistical inference, and hypothesis testing and generation. Class size is approximately 140 PharmD students.
Students are encouraged to use OpenEpi, a free-online interface that can perform simple statistical inferences. Open-Epi was developed by researchers at the Rollins School of Public Health at Emory University from a grant by the Bill and Melinda Gates Foundation. You can find Open-Epi here. Source: Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version. www.OpenEpi.com, updated 2013/04/06, accessed 2018/01/21.
Audio recordings of my slides are available here.
Video lecture of the biostatistics refresher is located here.
PDF versions of my slides are available here.
Optional readings that provide more details from the lectures are available here.
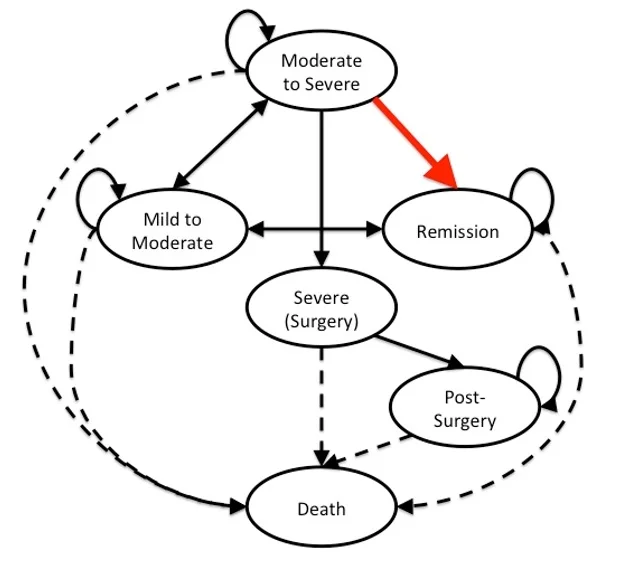
Markov model of Crohns' disease. The red arrow indicates the transition from the Moderate-to-Severe health state to the Remission state. The solid arrows indicates the transition states in the model. The dashed arrows indicates the transitions to the absorbing state (Death).
PHARM 536 - Advanced Methods in Economic and Outcomes Evaluation in Health and Medicine (Josh J. Carlson, University of Washington)
Schedule: Spring 2017
Description: Covers advanced methods and techniques for evaluating costs, outcomes, and cost-effectiveness of health, medical, and pharmaceutical interventions. Topics include: network meta-analysis, Markov modeling, probabilistic sensitivity analysis, value of information analysis, utility mapping, conjoint analysis, and budget impact analysis.
Summary: I helped teach a lecture in this class with my dissertation committee chair, Dr. Beth Devine. The lecture reviewed my work on generating evidence for a transition probability in a Markov model simulating Crohns' disease. Using a network meta-analysis framework, I was able use Bayesian methods to determine the transition probability of going from the Moderate-to Severe health state to the Remission state (red arrow). The class size was approximately 10 graduate students (PhD, MPH) and fellows.
The PowerPoint slides to my lecture can be downloaded here.
PHARM 534 - Economic Evaluation in Health and Medicine (Louis P. Garrison and David L. Veenstra, University of Washington)
Schedule: Fall 2017
Description: Methods and techniques for evaluating costs and cost-effectiveness of health, medical, and pharmaceutical interventions. Emphasis on economic evaluation, decision analysis, and modeling techniques for resource allocation and decision making. Applications to technology assessment, health policy, clinical practice, and resource allocation.
Summary: I lectured on costs accounting methods for Dr. David L. Veenstra, who was unable to attend class. One of the updates that I contributed to the lecture was the use of the Federal Supply Schedule (FSS) pricing instead of the traditional AWP/WAC pricing. The Second Panel on Cost-Effectiveness in Health and Medicine recommends FSS pricing when possible over AWP/WAC pricing. The was predominantly a PharmD class with approximately 30 students.
Pharm 541 - Pharmacy, Healthcare, and Society (Sean D. Sullivan, University of Washington)
Schedule: Winter 2016
Description: Introduction to health services and pharmacy practice designed for future healthcare practitioners. Examines the history, organization, and effectiveness of the U.S. healthcare system. Stresses the student's ability to adopt a broad perspective across healthcare disciplines and traditional boundaries.
Summary: I was the teaching assistant for PHARM 541 for the Winter 2016 Quarter. I helped write questions and grade exams for Dr. Sean D. Sullivan. This was predominantly a PharmD class with approximately 140 students.